
The electrochemical reduction (CO2-RR) of CO2 into high value-added industrial products through electrical energy enables the chemical storage of electrical energy, by which CO2 can be turned into a treasure. The reduction of CO2 content increases its commercial value at the same time.
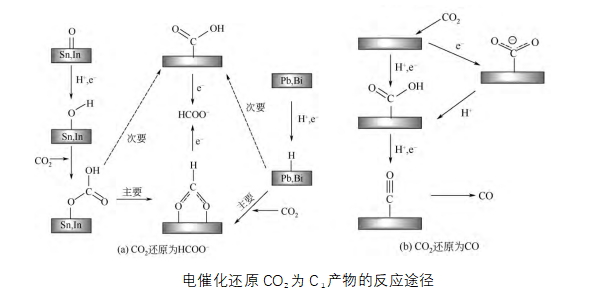
CO2 is the end product of organic combustion, and its chemical state is quite stable, so the energy barrier of activated CO2 molecules is high, and a high overpotential is required to induce the electrocatalytic reaction to occur. On the other hand, the electrochemical CO2 reduction reaction involves a multi-electron transfer mechanism, and a variety of products such as carbon monoxide, formate, methanol, ethylene and ethanol can be generated under different reaction conditions. The slow chemical reaction rate and the accompanying side reactions are the main obstacles to the effective reduction of CO2 to specific products. The factors affecting the electrochemical reduction of CO2 include electrode potential, electrocatalytic material, electrolyte solution and pressure temperature, among which the electrocatalytic material plays a decisive role in the product distribution and selectivity of the reduction reaction. So far, the effective catalysts that can electrochemically reduce CO2 to hydrocarbons and alcohols are still overwhelmingly copper-based materials.
1、Metal nanocatalysts
Compared with traditional bulk metals, nanostructured metal electrodes improve the specific surface area and increase the number of catalytic active sites on the one hand; on the other hand, the nanocatalyst surface has a large number of ligand unsaturated sites, which can significantly increase the catalytic activity and improve the electrocatalytic performance. The size, structure and morphology of metal nanocatalysts have a very important influence on the efficiency and product distribution of CO2 electrochemical reduction reaction. Electrochemical experiments show that the selectivity of H2 and CO increases with decreasing particle size, especially below 5 nm, while the selectivity of CH4 and C2H4 decreases.
2、Metal alloy catalyst
The electronic properties of individual atoms in a substance are affected by nearby atoms. After doping monometallic materials with other metallic elements, the interaction between the atoms of different elements will significantly change their electronic structures, which in turn will change the adsorption strength of intermediates on the catalyst surface; in addition, changes in the arrangement of atoms at the active site will also change the adsorption of intermediates with the surface. For example, Cu-In alloy catalysts were prepared by electrodeposition of indium onto a rough copper surface for the electrocatalytic reduction of CO2. Although the current density of the alloy catalysts did not increase significantly compared to the monometallic Cu electrode without In deposition, the alloy catalysts inhibited the occurrence of hydrogen precipitation reactions and significantly improved the selectivity of CO. At a potential of -0.7 V, the FE for the conversion of CO2 to CO reached 95%, much higher than that of monometallic Cu or In electrodes.
3. Oxide derived metal catalysts
Pre-oxidation of metals followed by in situ electrochemical reduction during the reaction is another method to modify metal electrodes to improve their catalytic performance in CO2 electrochemical reduction reactions. It was reported that by increasing the oxidation temperature and growing the oxidation time, the Cu foil was annealed in air to obtain a nanowire-shaped catalyst with a Cu2O layer on the surface, and this catalytic material reduced the overpotential of electrochemical reduction of CO2 by about 0.5 V compared to that of polycrystalline Cu foil.
4. Metal-based composite catalysts
In the field of electrocatalysis, metal materials are most widely studied, but common metal electrodes still have disadvantages such as low current efficiency and poor stability in the process of CO2 reduction. Carbon materials with high specific surface area and electrical conductivity are often used as catalyst carriers due to their easy modification and functionalization. Loading metals onto heteroatom-doped carbon materials, the interaction between the metal and the carrier can change the electronic structure of the metal, which in turn can improve its intrinsic catalytic activity, facilitate the activation of CO2 molecules and adjust the binding energy of important intermediates to the catalyst, and is an efficient method to improve the catalytic performance of CO2 reduction at metal electrodes. It has been reported that a copper-based composite (Cu/CNS) was prepared by loading copper nanoparticles onto multilayer graphene (CNS) with a large number of folds and spikes and doped with nitrogen by an electrochemical nucleation method. exhibited superior catalytic performance: the FE of C2H5OH was as high as 63% at a potential of -1.2 V, while almost no C2H5OH was generated by the former two, and the current densities were 3 and 5 times higher than the former two, respectively, and the activity and selectivity of the Cu/CNS catalyst did not decrease significantly after a continuous reaction of 6 h.
5. Single-atom catalysts
As the material continues to shrink from the nanoscale to the individual atomic level, its geometric and electronic structure changes radically, thus exhibiting completely different catalytic properties from those of nanomaterials. Using the ion exchange method, nickel precursors are dispersed onto the ZIF-8 surface with zinc as the attachment site and then heated at high temperature (1000°C); since the boiling point of Zn is 907°C, the Zn nodes evaporate during the heating process and leave defects, and these sites are easily occupied by neighboring nickel atoms, thus forming Ni single-atom catalysts (NiSAs/N-C); in the electro chemical reaction, this NiSAs/N-C catalyst converted CO2 to CO with an FE of about 71.9% and a current density of 10.48 mA/cm2, and showed good stability in a continuous reaction of 60 h. Most current single-atom catalytic materials produce mainly CO in the electrochemical reduction reaction of CO2, and the design of single-atom catalysts capable of reducing CO2 to hydrocarbons or alcohols still faces a great challenge.
Catalytic materials for the electrochemical reduction of CO2 have been developed in recent years, and the electrocatalytic performance of the new metal-based materials has made great progress compared to the traditional polycrystalline metal electrodes. However, the high overpotential requirement, low product selectivity (especially for direct reduction of CO2 to hydrocarbons or alcohols) and unsatisfactory reaction stability make metal-based electrocatalytic materials far from the standard for industrial applications, and thus the development of higher performance electrocatalytic materials remains one of the main directions for future research in the field of CO2 electrochemical reduction.

Anhui Yuanchen Environmental Technology Co., Ltd (hereinafter referred to as "Yuanchen Technology") is a high-tech enterprise integrating R&D, production and sales of dust removal bags and denitrification catalysts. Over the past sixteen years, Yuanchen Technology has been focusing on the environmental protection field, and now has 4 international PCT, 33 authorized invention patents and 77 invention patents in process, among which many products such as "dust removal and denitrification integrated technology products" have been awarded the Scientific Progress Award of Anhui Province. Our dust removal bags (mainly PPS, PTFE, P84 and composite series filter materials) and SCR denitrification catalysts have been widely used in cement, steel, glass kilns, waste incineration power generation, biomass power generation, non-ferrous metal smelting and other industries.
In the future, Yuanchen Technology will be guided by "becoming the guardian of global ecological environment", always rooted in environmental protection, and insist on the great cause of guarding the blue sky and white clouds. Leveraging on the national ecological civilization construction pattern, we will continue to deepen technology, optimize management, strengthen brand, refine industry and solidify advantages, and create synergistic value for industry through comprehensive and integrated governance and services.