
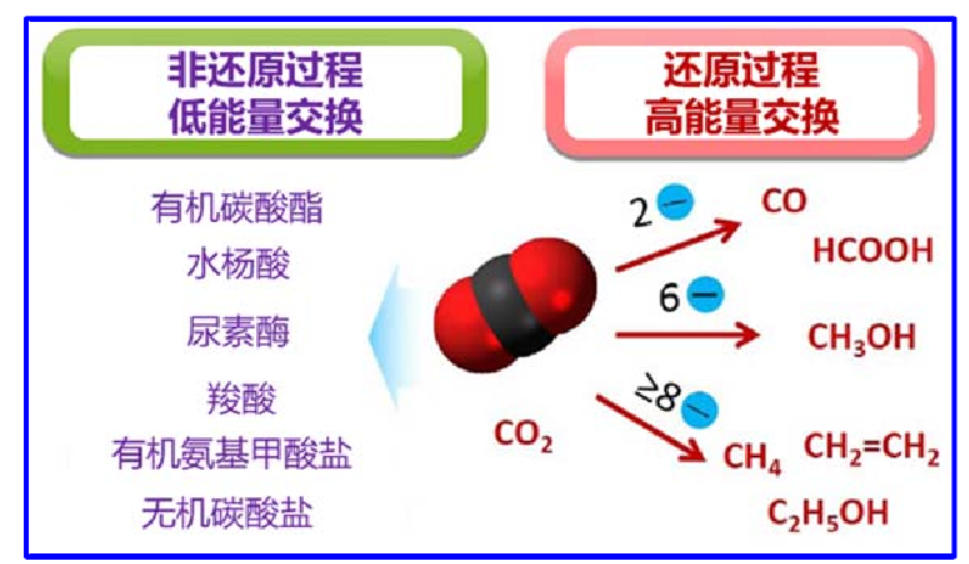
At present, the research on conventional thermocatalytic reduction of CO2 mainly focuses on the development of highly active, selective and stable catalytic materials and the catalytic mechanism, and the products are mainly carbon monoxide (CO), methanol (CH3OH) low carbon olefins and hydrocarbon fuels. CO production by reverse water gas (RWGS) reaction is highly flexible because the product CO can be reused as reactants for methanol and hydrocarbon production. However, RWGS reactions are typically heat-absorbing and require higher temperature conditions; therefore, research on RWGS reactions has focused on how to overcome reaction kinetic barriers to obtain higher CO yields; conversion of CO2 to methanol is the most direct route for CO2 utilization, as methanol can be used directly as a fuel additive, fuel substitute, and precursor for many general purpose chemicals. Although the synthesis of methanol from CO2 by hydrogenation is an exothermic process, the reaction is again limited by kinetic factors at low temperatures.
Two main pathways for the conversion of CO2 into different products
1、Reverse water gas reaction to produce carbon monoxide
Typical RWGS reaction catalysts usually have the active component highly dispersed on the metal oxide carrier surface to improve the catalyst activity by increasing the metal-carrier interface. Studies have shown that two different reaction paths may exist for the RWGS reaction to produce CO, one is the redox mechanism (taking the active metal Cu as an example), in which the CO2 on the catalyst surface oxidizes CuO to Cu+ and generates CO, and then H2 reduces Cu+ to CuO and generates H2O; the other more widely recognized is the formate decomposition mechanism, in which CO2 is firstly generated by hydrogenation to formate Therefore, an efficient RWGS catalyst should have both good hydrogenation activity and C=O double bond breaking activity. Among them, metal oxide catalysts loaded with metal particles are widely used due to the better H2 dissociation ability of the metal and the hydrogen overflow effect between the metal and the carrier. Based on the above proposed reaction mechanism, the commonly used catalyst systems are Cu-based catalysts, noble metal catalysts and catalysts with CeO2 as the carrier.
2、CO2 hydrogenation to methanol
The hydrogenation of CO2 to methanol (CAMERE) by RWGS reaction can achieve a yield of about 75 Mt per year. The whole process first requires the RWGS reaction on ZnAl2O4, and after removing the water gas, the reaction of methanol synthesis on Cu/ZnO/ZrO2/Ga2O3. Although the process can obtain a certain amount of methanol, it requires two kinds of catalysts and reactors, which has a high cost. Therefore, the current study focuses on the direct hydrogenation of CO2 to methanol over Cu-based catalysts. The results show that the catalytic activity of Cu-based catalysts is significantly structure sensitive, and different elemental compositions, crystal surface exposure, and interfacial structures mainly affect the CO2 adsorption and activation processes, which in turn affect the methanol selectivity and yield. On the basis of mechanistic studies, many studies have also enhanced the catalysts' performance on CO2 adsorption and activation and the selectivity of the product methanol by adding additives or adopting other catalytic systems.
3. Fischer-Tropsch reaction to produce hydrocarbons
Another pathway of CO2 catalytic reduction is the production of alkanes or olefins through the Fischer-Tropsch reaction (CO2-FT) of CO2 hydrogenation. Currently, 200Mt of olefins are produced each year while 1.2-1.8 tons of CO2 are emitted. The CO2-FT route can reduce CO2 emissions while preparing olefins. However, the design of high activity CO2-FT catalysts is very difficult. The ideal catalyst must have both high RWGS reaction activity and high FT reaction activity, and the thermodynamic limits of the RWGS reaction can only be broken if the FT reaction process can convert the CO generated by the RWGS reaction fast enough to achieve higher CO2 conversion. The commonly used catalysts for the conventional FT reaction via syngas (CO-FT) are mainly Fe-based catalysts (at high temperature) and Co-based catalysts (at low temperature), and a comparison of their catalytic activities for CO-FT and CO2-FT shows that the conversion of CO (up to 87%) is significantly higher than that of CO2 (up to 45%), which indicates that when the carbon source of the reaction is changed from CO to CO2, the new catalysts need to be designed and developed based on the conventional FT reaction catalysts to improve the reaction activity. The current research on CO2-FT reaction catalysts is dominated by Fe-based catalysts, which have higher CO2 conversion and higher carbon-olefin selectivity than Co-based catalysts, and the improved performance of Fe-based catalysts is mainly caused by the improved activity of RWGS reaction. There is still a large disagreement about whether the active center of the Fe-based catalyst is iron oxide or ferrous oxide during the reaction. In addition to the production of high carbon olefins, the direct methanation of CO2 is also very important to be studied in some specific regions.The study of CO2 methanation reaction is dominated by Ni-based catalysts, while some other noble metals (e.g. Ru, Rh) show higher low-temperature catalytic activity.
The most important issue of CO2 catalytic reduction is the selectivity, and to improve the selectivity of specific products requires a comprehensive consideration of the kinetics, thermodynamics and intermediates of the reaction. There are still many problems in the design of highly active, selective and stable catalysts for different CO2 reduction pathways, as follows: (1) stabilization of the key intermediates of the reaction on the catalyst surface; (2) characterization of the reaction process and study of the mechanism by in situ means; (3) development of low-cost catalysts; (4) improvement of the catalysts' resistance to water and poisoning; (5) development of CO2 emission-free new H2 source without CO2 emission, etc. Among them, the characterization and mechanism of the reaction process by in-situ means is the key to solve the problem.

Anhui Yuanchen Environmental Technology Co., Ltd (hereinafter referred to as "Yuanchen Technology") is a high-tech enterprise integrating R&D, production and sales of dust removal bags and denitrification catalysts. Over the past sixteen years, Yuanchen Technology has been focusing on the environmental protection field, and now has 4 international PCT, 33 authorized invention patents and 77 invention patents in process, among which many products such as "dust removal and denitrification integrated technology products" have been awarded the Scientific Progress Award of Anhui Province. Our dust removal bags (mainly PPS, PTFE, P84 and composite series needle felt) and SCR denitrification catalysts have been widely used in cement, steel, glass kilns, waste incineration power generation, biomass power generation, non-ferrous metal smelting and other industries.
In the future, Yuanchen Technology will be guided by "becoming the guardian of global ecological environment", always rooted in environmental protection, and insist on the great cause of guarding the blue sky and white clouds. Leveraging on the national ecological civilization construction pattern, we will continue to deepen technology, optimize management, strengthen brand, refine industry and solidify advantages, and create synergistic value for industry through comprehensive and integrated governance and services.